Introduction
Brentuximab vedotin (BV), a CD30-directed monoclonal antibody, and programmed cell death-1 inhibitors (PD-1; e.g., nivolumab, pembrolizumab) are efficacious for the treatment of adult patients with Hodgkin lymphoma (HL) at high risk of relapse or progression post-autologous stem cell transplantation (ASCT). In this study, we investigated the real-world treatment patterns and outcomes of patients with relapsed/refractory HL (RRHL) who had received ≥1 line of therapy (LOT).
Methods
Patients ≥18 years old with evidence of RRHL in ≥1 inpatient or ≥2 outpatient physician medical notes or evidence of ≥1 autologous/allogeneic hematopoietic stem cell transplant (HSCT) procedure following evidence of HL in the Humedica Electronic Medical Record (EMR) database, were selected between October 1, 2016 and September 30, 2020. The earliest evidence of RRHL (medical note or HSCT procedure) was defined as the patient's index date. Patients were excluded if they had evidence of RRHL, non-Hodgkin's disease, other primary cancers, or metastatic disease in the 12 months prior to the index date (baseline period). Patient demographic and clinical characteristics were evaluated during the baseline period and the index date. Patients were followed from their index date until patient death or the end of recorded healthcare activity during which treatment patterns and outcomes were assessed.
Results
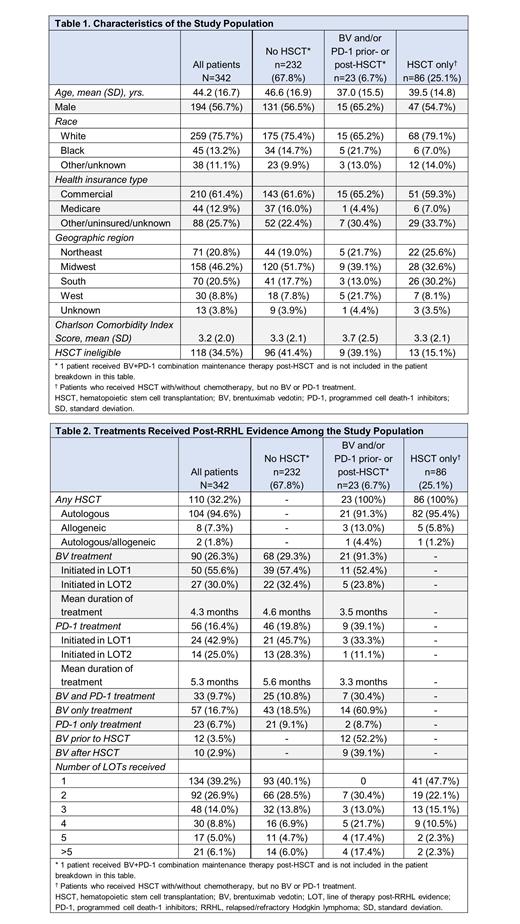
A total of 342 patients with ≥1 LOT post-RRHL during the follow-up period were included in the final study population. Mean age was 44.2 years, 56.7% were male, 75.7% were white, and 61.4% had commercial insurance; mean Charlson Comorbidity Index score was 3.2 (Table 1). Approximately one-third (34.5%) of patients were not eligible for HSCT based on available laboratory data and comorbidity diagnoses during the baseline period or the index date. Across the study population, the average follow-up duration was 25.6 months (median: 23.9 months). Among the overall study population, 90 (26.3%) patients received BV treatment, with most (~86%) receiving BV in LOTs 1 and 2 post-RRHL evidence (Table 2). The average time from index date to initiation of BV treatment was 2.5 months and mean duration of treatment (DOT) with BV was 4.3 months. Most patients treated with BV (n=68) had not received a HSCT. During the follow-up, among those who received BV but not a PD-1 (n=57), the progression-free survival (PFS) rate was 73.7% and overall survival (OS) was 82.5%. Among the overall study population, 56 (16.4%) patients received a PD-1, with most patients (~68%) receiving a PD-1 in LOTs 1 and 2 post-RRHL evidence. The average time from index date to initiation of PD-1 treatment was 5.5 months and mean DOT with a PD-1 was 5.3 months. Among those patients who received a PD-1 but not BV (n=23), the PFS rate was 56.5% and OS was 69.6%. One patient received post-HSCT BV+PD-1 combination maintenance therapy. Among the patients who received BV and/or a PD-1 prior- or post-HSCT, 12 (52.2%) received BV prior to the HSCT and 9 (39.1%) received BV after the HSCT. Among those who received BV and PD-1 treatments (i.e., not at the same time; n=33), the PFS rate was 60.6% and OS was 90.9%. Among the study population, a majority of patients (n=232; 67.8%) did not receive a HSCT during the follow-up period (including the index date). Of these patients without a HSCT, 68 (29.3%) received BV, 46 (19.8%) received a PD-1, and 25 (10.8%) received both BV and a PD-1 (i.e., not at the same time).
Conclusions
In this real-world evaluation of the treatment patterns and outcomes among patients in post-RRHL evidence setting, BV was administered more frequently in patients without HSCT. Increasing the use of BV in patients with ASCT at high risk for relapse or progression may lead to improved clinical outcomes. This study was based on EMRs from a single administrative database and has a limited sample size; these findings may not apply to all patient populations in post-RRHL evidence setting in the US.
Disclosures
Kristo:Takeda: Current Employment, Current equity holder in publicly-traded company. Lingohr-Smith:Takeda: Research Funding. Lin:Takeda: Research Funding. Ashaye:Pfizer Inc: Current equity holder in publicly-traded company; Merck: Current equity holder in publicly-traded company; JNJ: Current equity holder in publicly-traded company; 2Seventy Bio: Current equity holder in publicly-traded company; CNS pharmaceuticals Inc: Current equity holder in publicly-traded company; Enveric Biosciences Inc: Current equity holder in publicly-traded company; Evogene Ltd: Current equity holder in publicly-traded company; Genetic Technologies: Current equity holder in publicly-traded company; Eli Lily: Current equity holder in publicly-traded company; Sanofi: Current equity holder in publicly-traded company; Seagen Inc: Current equity holder in publicly-traded company; Takeda Pharmaceuticals Inc.: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal